Long COVID
Long COVID is when people have symptoms for a long time after they had COVID-19, even after the virus is gone. Some people can feel tired, have trouble breathing, difficulty concentrating, or feel pain in different parts of their body for weeks, months, or even years.1 Long COVID can be challenging since each patient has a different experience with symptoms and how it affects their daily life.
- For Patients
- For Physicians
Background
Long COVID is when people have symptoms for a long time after they had COVID-19, even after the virus is gone. Some people can feel tired, have trouble breathing, difficulty concentrating, or feel pain in different parts of their body for weeks, months, or even years.1 Long COVID can be challenging since each patient has a different experience with symptoms and how it affects their daily life.
Who can get Long COVID?
Anyone who had COVID-19 can get long COVID. It does not matter if they had severe symptoms or only felt a little sick at first. Sometimes, people who didn’t even know they had COVID and did not have any symptoms (or were asymptomatic) can still get long COVID.2
How many people have it?
A lot of people have had COVID-19 — over 650 million worldwide! It is estimated that about 1 out of every 10 people who had COVID-19 might have long COVID, which could mean that about 65 million people are experiencing symptoms of long COVID.3
Since long COVID is still not recognized by all people, symptoms may be ignored or mistaken for other conditions.
Many patients also report co-occurring conditions such as mast cell activation syndrome, chronic fatigue syndrome, autoimmune diseases, postural orthostatic tachycardia syndrome (POTS), and many others.4-6
Symptoms1
People with long COVID can feel lots of different things, like:
- Feeling tired (fatigue)
- Difficulty breathing
- Heart racing
- Pain in muscles or joints
- Changes in smell or taste
- Headaches
- Coughing
- Trouble thinking clearly (brain fog)
- Trouble sleeping
- Upset stomach or diarrhea
These symptoms can come and go and sometimes make it hard to do everyday things.
Treatment Options
Right now, doctors don’t have a cure for long COVID. Doctors can help by trying to manage the symptoms. This can include medicine, physical therapy, help with thinking and memory problems, and support for mental health. Scientists are still learning the best ways to treat long COVID.
How is SAFIRE helping?
People living with long COVID have reported that they have gotten limited advice, different answers from different providers, or they report feeling dismissed by healthcare professionals.7 Sadly, there are still things about long COVID that are not fully understood, and a lot of patients are unable to get care for long COVID, have symptoms that are not recognized, or are given treatments that do not improve their symptoms.
SAFIRE is working to raise money for research to learn more about long COVID and how to treat it better. We also want to give patients and doctors better information and resources.
If you want to help SAFIRE, please consider making a contribution on our donation page.
Integrated Pathophysiological Loop in Long COVID
Dr. Vaughn, a leading doctor and researcher in long COVID, has come up with a new theory to explain what might be happening in the bodies of people who suffer from long COVID and similar illnesses like myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
In long COVID and ME/CFS, a harmful cycle starts in the body’s small blood vessels. Tiny blood clots, or “microclots”, form and don’t break down the way they normally should. These microclots are made of sticky proteins (called fibrin/fibrinogen) and trapped chemicals from inflammation. They block the smallest blood vessels, which makes it harder for oxygen and nutrients to reach the body’s tissues.

Some of these microclots also block special small blood vessels that feed the walls of larger veins. When this happens, the vein walls don’t get enough oxygen and begin to break down. The veins become weaker, stretched out, and lose their normal shape. As a result, the valves inside the veins stop working well, and nearby structures or body parts can press on these weak veins. This can lead to conditions like May-Thurner Syndrome, where a vein gets compressed in the pelvis, and other pelvic venous disorders.
As the veins stretch and get compressed, they don’t work as well—especially when a person stands up. Blood starts to collect in the lower body instead of returning to the heart. The heart doesn’t get enough blood to pump out to the rest of the body, especially during movement or when standing. This problem is called preload failure.
To make up for this, the body tries to help by making the heart beat faster and by breathing faster. But breathing too fast can cause carbon dioxide (a gas we naturally breathe out) to drop too low. Carbon dioxide helps control how much blood flows to the brain, so when there isn’t enough of it, the brain’s blood vessels tighten, and the brain doesn’t get enough blood. This leads to symptoms like feeling dizzy, tired, confused, or having “brain fog.”
Meanwhile, the same microclots are also blocking blood flow in the muscles. This makes it hard for the muscles to use oxygen properly, so they switch to a less efficient way of making energy. This creates lactic acid, makes people feel tired more quickly, and causes them to breathe even faster, continuing the cycle of low carbon dioxide.
The muscles, especially in the legs, also help push blood back up to the heart when we move or stand. But when the muscles get weaker, they can’t do this job well, which makes the blood pooling and heart problems even worse.
And finally, when blood stays still in stretched or compressed veins, more microclots can form, starting the whole cycle over again.
Dr. Vaughn’s theory connects problems with blood vessels, muscles, and the nervous system to explain how long COVID and ME/CFS might create a lasting pattern of illness in the body.
References
- Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–442.
- Elizabeth T. Cirulli KMSB, Stephen Riffle, Alexandre Bolze, Iva Neveux, Shaun Dabe, Joseph J. Grzymski, James T. Lu, Nicole L. Washington. Long-Term COVID-19 Symptoms in a Large Unselected Populaiton. medRxiv. 2020.
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146.
- Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. 2021;112:217–226.
- Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414.
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of Long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673.
- Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144.
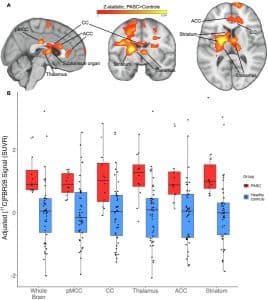
- VanElzakker MB, Bues HF, Brusaferri L, Kim M, Saadi D, Ratai EM, Dougherty DD, Loggia ML. Neuroinflammation in post-acute sequelae of COVID-19 (PASC) as assessed by [11C]PBR28 PET correlates with vascular disease measures. Brain Behav Immun. 2024 Jul;119:713-723. doi: 10.1016/j.bbi.2024.04.015. Epub 2024 Apr 18. PMID: 38642615; PMCID: PMC11225883.
- Pretorius, E., Vlok, M., Venter, C. et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 20, 172 (2021). https://doi.org/10.1186/s12933-021-01359-7
Background
Long COVID, also referred to as Post-Acute Sequelae of COVID-19 (PASC), is a multifaceted syndrome characterized by a constellation of symptoms persisting or emerging beyond the acute phase of SARS-CoV-2 infection. The heterogeneity of clinical manifestations, symptom severity, and functional impairment presents a significant diagnostic and therapeutic challenge. Long COVID may affect patients regardless of initial disease severity, including those with asymptomatic infection.1
Commonly reported symptoms include profound fatigue, dyspnea, chest pain, arthralgia, cognitive impairment, musculoskeletal pain, and others.2 The pathophysiology is not fully understood but people have hypothesized that it is multifactorial, involving immune dysregulation, autonomic dysfunction, microvascular injury, potential viral persistence, and several other factors.
Estimates suggest approximately 10% of individuals infected with SARS-CoV-2 experience long-term sequelae, implicating a substantial global burden given the extensive incidence of COVID-19 cases worldwide. Based on this percentage, it can be estimated that there are likely 65 million individuals with symptoms of long COVID.3
Patients with long COVID frequently present with overlapping syndromes such as mast cell activation syndrome (MCAS), myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), autoimmune phenomena, and postural orthostatic tachycardia syndrome (POTS), complicating diagnosis and management.4-6
Clinical Presentation2
Long COVID symptomatology is broad and variable, including but not limited to:
- Severe fatigue and post-exertional malaise
- Dyspnea and exertional intolerance
- Palpitations and chest pain
- Olfactory and gustatory disturbances
- Myalgias and arthralgias
- Persistent cough
- Headaches
- Gastrointestinal symptoms such as diarrhea
- Cognitive dysfunction (“brain fog”)
- Sleep disorders and autonomic symptoms
Symptom severity often fluctuates, leading to significant impairment in activities of daily living and reduced quality of life.

Currently, evidence-based therapies for long COVID remain limited. Treatment paradigms focus predominantly on symptom-targeted approaches, including pharmacologic management (e.g., analgesics, autonomic agents), physical rehabilitation, cognitive therapy, and psychosocial support. Multidisciplinary care models are advocated given the complexity and multisystem involvement. Ongoing clinical trials and research are critical to elucidate pathogenetic mechanisms and develop targeted therapeutics.
Clinicians should be vigilant for comorbid conditions and secondary diagnoses, ensuring a comprehensive and individualized approach to care.
Recently a link between symptoms of long COVID and increase venous pooling and vascular resistance have been suggested. Iliac venous stenting is currently under investigation as a potential treatment option for patients with long COVID (see below theory from Dr. Vaughn).
Role of SAFIRE
Patients with long COVID often report inconsistent medical advice and a perceived lack of validation from healthcare providers, underscoring the urgent need for increased awareness and specialized care pathways.7 SAFIRE is committed to advancing research on long COVID pathophysiology and treatment, with a focus the link between symptoms and pelvic venous disorders. By funding research and expanding educational resources, SAFIRE seeks to improve diagnostic accuracy and therapeutic outcomes for affected patients.
If you are interested in supporting SAFIRE’s mission, please consider contributing through our donation page.
Integrated Pathophysiological Loop in Long COVID
Dr. Vaughn–a leading physician and researcher in the long COVID space–has developed the following integrated theory for the pathophysiological loop seen in long COVID patients.
In long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a complex and self-reinforcing cycle of systemic dysfunction unfolds, beginning at the vascular and microvascular level. One of the earliest triggers in this cascade appears to be the formation of persistent, fibrinolysis-resistant microclots that are composed of fibrin(ogen), amyloid, and trapped inflammatory molecules. These microclots not only impair oxygen and nutrient delivery at the capillary level but also obstruct the vasa vasorum—the small vessels that supply the walls of larger veins.

When the vasa vasorum are blocked, the tunica media of veins becomes ischemic. This critical layer, composed of smooth muscle, begins to degenerate due to hypoxia, leading to venous wall weakening, dilation, and loss of tone. As venous valves lose competency and the vessel loses mechanical resilience, these veins become more susceptible to external compression by adjacent anatomical structures—a mechanism that can give rise to clinically recognizable venous compression syndromes, such as May-Thurner Syndrome or other pelvic venous disorders.
As these veins become progressively dilated and compressed, they begin to exhibit venous insufficiency, especially in upright positions. Blood pools in the lower body, reducing venous return to the heart, which results in preload failure—a condition in which the heart receives insufficient blood to pump effectively during stress or posture changes. This leads to a decrease in stroke volume, particularly noticeable during physical exertion or orthostatic stress.
To compensate for the low cardiac output, the body increases heart rate and ventilation rate in an attempt to deliver more oxygen systemically. However, this hyperventilation is not matched by metabolic demand, leading to a drop in arterial carbon dioxide levels—hypocapnia. Carbon dioxide is a major regulator of cerebral blood flow; thus, when it drops too low, the brain’s blood vessels constrict, resulting in cerebral hypoperfusion.
This state of Hypocapnic Cerebral Hypoperfusion (HYCH) causes hallmark symptoms of long COVID and dysautonomia, including lightheadedness, brain fog, cognitive dysfunction, and fatigue. Simultaneously, the same microclots and inflammatory processes that began the loop continue to obstruct capillaries in skeletal muscle. This impairs oxygen extraction and mitochondrial oxidative phosphorylation, reducing muscle efficiency and causing early anaerobic metabolism, lactic acid accumulation, and increased ventilatory drive—which further perpetuates hypocapnia.
Muscle dysfunction also reduces the effectiveness of the skeletal muscle pump, an essential mechanism for propelling venous blood back to the heart during upright activity. The loss of pump efficiency worsens venous pooling and exacerbates preload failure, reinforcing the cycle.
At the same time, venous stasis from compression and dilated veins promotes further microclot formation, feeding back into the original trigger of the cycle: vascular occlusion, endothelial dysfunction, and thromboinflammation.
This loop reflects a unified mechanism by which vascular, muscular, and autonomic systems become entangled in a chronic dysfunction cycle seen in long COVID, ME/CFS, and related syndromes.
References
- Elizabeth T. Cirulli KMSB, Stephen Riffle, Alexandre Bolze, Iva Neveux, Shaun Dabe, Joseph J. Grzymski, James T. Lu, Nicole L. Washington. Long-Term COVID-19 Symptoms in a Large Unselected Populaiton. medRxiv. 2020.
- Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–442.
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146.
- Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. 2021;112:217–226.
- Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16(8):413–414.
- Pollack B, von Saltza E, McCorkell L, et al. Female reproductive health impacts of Long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front Rehabil Sci. 2023;4:1122673.
- Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144.